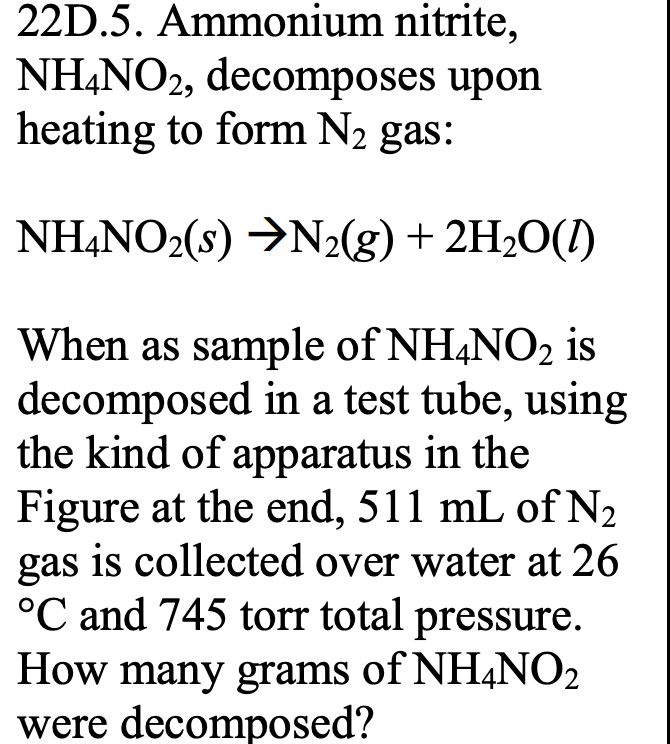
Ammonium Nitrite Decomposes to Nitrogen Gas and Water
B Calculate the quantity in grams of NH4NO2 needed to inflate a tennis ball to a volume of 862 mL at 120 atm and 22 degree C. In this decomposition reaction NH4NO3 is breaking apart decomposing into N2 O2 H2O.
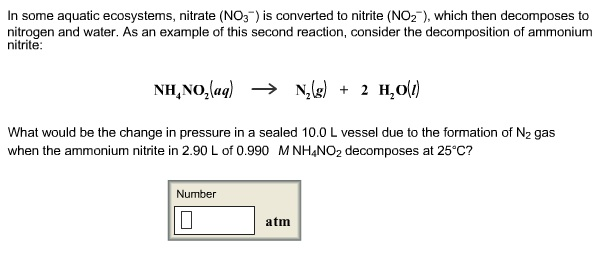
Solved In Some Aquatic Ecosystems Nitrate No3 Is Chegg Com
Ammonium nitrate nh4no3 decomposes into nitrogen chegg com general reactions dr ron rusay chemical 1 the balanced reaction equation for solved is highly explosive if not handled carefully breaking down gas oxygen and thermal decomposition.
. Ammonium nitrate decomposes into the gases nitrous oxide and water vapor when heated not an explosive reaction. Type of Chemical Reaction. Energy left the system so the reaction is endothermic.
This property is used to inflate some tennis balls. Write a balanced chemical reaction describing the decomposition of ammonium nitrate and calculate the standard heat of reaction using the. Does nitrate react with water.
However it can be induced to decompose explosively by detonation. 2NH4NO3s - - 2N2g 4H2Og O2g How many grams of NH4NO 3 are needed to produce 30 L of O 2 gas. Ammonium nitrate Nitrogen gas Oxygen gas Water.
This equation isNH4NO2 N2 2 H2O. How many grams of dry N H 4 N O 2 s were initially decomposed. Write a balanced chemical reaction describing the decomposition of ammonium nitrate and calculate the standard heat of reaction using the appropriate enthalpies of formation.
Ammonium nitrite decomposes upon heating to form nitrogen gas and water vapor according to the following unbalanced chemical reaction. Include the steps that you took. Ammonium nitrite NH4NO2 will decompose to yiel nitrogen gas and water Vapor by the following NH4NO2 s ------ 2H2O g N2 g This 14 words question was answered by Heather L.
N2 g H2Og Now the Law of Mass Conservation tells you that matter can neither be created nor destroyed in a chemical reaction. In alcohol fermentation yeast converts glucose to. Write a balanced equation for the reaction.
Ammonium nitrite NH 4 NO 2 decomposes upon heating to form N 2 gas. Ammonium nitrate decomposes to N2O and water at temperatures between 250 C and 300 C. Nitrogen gas does not react with water.
A Write a balanced equation for the reaction. What is the balanced equation of solid ammonium nitrite decomposes to liquid water and nitrogen gas. The temperature decreased when the ammonium nitrate dissolved in the water.
This property is used to inflate some tennis balls. Ammonium nitrate decomposes to N2O and water at temperatures between 250 C and 300 C. When ammonium nitrite NH 4 NO 2 is heated it decomposes to liquid water and nitrogen gas.
The ionic bonds between the NH4 ions and NO-3 ions were broken when the ammonium nitrate dissolved in the water. Ammonium nitrate will decompose explosively at high temperatures to form nitrogen oxygen and water vapor. This property is used to inflate some tennis balls.
The global Ammonium Nitrate Market size was calculated at USD 141 Billion in 2021 and is estimated to achieve USD 167 Billion by 2028 with a CAGR of 28 during the forecast period of 2022-2028. Humidity is the amount of water vapor in the air at a given time. A Write a balanced equation for the reaction.
Total pressure of gas 745 torr Net pressure of gas total pressure. Ammonium nitrate decomposes to yield nitrogen gas water and oxygen gas in the following reaction. 2 mathrm NH _ 4 mathrm NO _ 3 rightarrow 2 mathrm N _ 2 mathrm O _ 2 4 mathrm.
This property is used to inflate some tennis balls. N H 4 N O 2 s N 2 g H 2 O g When a sample is decomposed in a test tube 511 m L of wet N 2 g is collected over water at 26 C and 745 torr total pressure. NH 4 NO 2s -- N 2g 2H2O When a sample of NH 4 NO 2 ois decomposed in a test tube 511 ml N 2 is collected over water at 26 C and 745 torr total pressure.
It does dissolve in water. NH4NO2 s Δaa. Youll need to be patient balancing this equation.
On StudySoup on 5312017. Calculate the quantity in grams of ammonium nitrite needed to inflate a tennis ball to a volume of 862 ml at 120 atm and 220C. When ammonium nitrite NH4NO2 is heated it decomposes to give nitrogen gas.
Calculate the mole fraction of water vapor in the gas mixture after 827 of the ammonium nitrate decomposes. This equation isNH4NO2 N2 2 H2O. Since its upload it has received 382 views.
The question contains content related to Chemistry and Science. When ammonium nitrite is heated it decomposes to give nitrogen gas. By admin January 14 2020.
When collecting a gas in the presence of water where the water that was a gas may be due to the energy associated with the reaction Has now condensed to a cooler temperature such as in this case 26. What is the balanced equation of solid ammonium nitrite decomposes to liquid water and nitrogen gas. Ammonium nitrite NH4NO2 will decompose to yield nitrogen gas and water vapor by the following equationNH4NO2 s 2H2O g N2 gA sample of 250g NH4NO2 was heated at 100 in a 100 L reaction vessel until all the NH 4NO2 had decomposed and H2O and N2 had formedAt the end of the reactiona what is.
Ammonium Nitrate Decomposes To Nitrogen Gas And Water Balanced Equation. How many grams of NH 4 NO 2 were decomposed. As you can see ammonium nitrite decomposes to form nitrogen gas N2 and water H2O according to the unbalanced chemical equation.
Ammonium Nitrate Market Overveiw. ASolid ammonium nitrate decomposes to form gaseous N 2 O and water vapor. 2 N H 4 N O 3 2 N 2 O 2 4 H 2 O.
A 0026 g sample of ammonium nitrate is placed inside an evacuated no gases present initially container. For this reaction we have a decomposition reaction. Ammonium nitrate decomposes explosively to form nitrogen oxygen and water vapor.
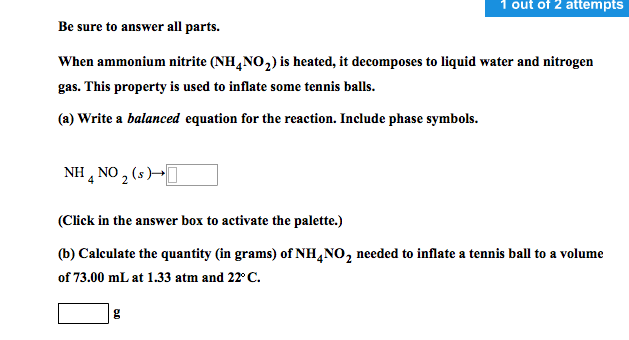
Solved Be Sure To Answer All Parts When Ammonium Nitrite Chegg Com

Nitrogen Fixation Definition Process Examples Types Facts Nitrogen Cycle Nitrogen Fixation Nitrogen

Biolincs Nitrogen Cycling In The Open Ocean Nitrogen Cycle Nitrogen Nitrogen Fixation

Water Free Full Text Nitrate Water Contamination From Industrial Activities And Complete Denitrification As A Remediation Option Html

How To Balance Nh3 O2 N2 H2o Ammonia Plus Oxygen Gas Youtube

Solved Ammonium Nitrite Decomposes Upon Heating To Form Nitrogen Gas And Water Vapor According To The Following Unbalanced Chemical Reaction Mathrm Nh 4 Mathrm No 2 S Longrightarrow Mathrm N 2 G Mathrm H 2 Mathrm O G When A
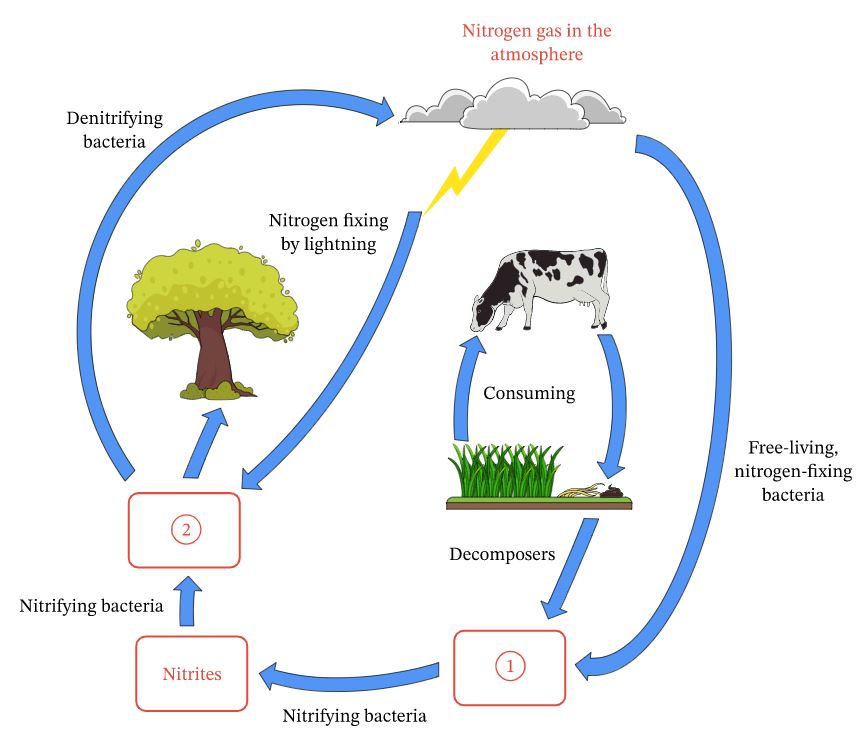
Nitrogen Cycle Science Quizizz

Intro To Chemical Reactions Chapter 6 How Do You Know If A Chemical Reaction Occurred We Look For Visual Signs Or A Chemical Change If Something Bubbles Ppt Download
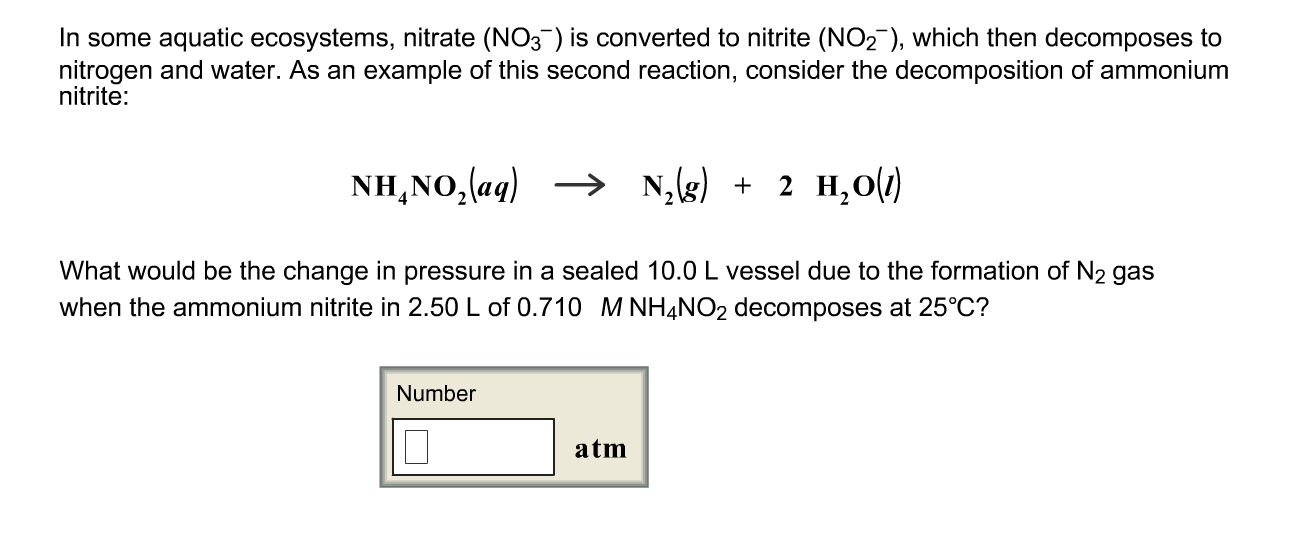
Solved In Some Aquatic Ecosystems Nitrate No 3 Is Chegg Com

How To Balance Nh4no3 N2 O2 H2o High Temperature Decomposition Youtube

Chemical Reactions Equations Ppt Download

Nitrate No3 Nitrite No2 Mobileh2o Llc
Solved 22d 5 Ammonium Nitrite Nh4no2 Decomposes Upon Chegg Com

Nitrogen Gas Can Be Obtained By Heating A Ammonium Nitrate B Ammonium Nitrte C Magnesium Nitride D Ammonium Chloride
N Free An For Nitrate And Ammonium Treatment 住友金属鉱山エンジニアリング株式会社
The Degradation Of Urea And The Nitrogen Cycle A A Simplification Of Download Scientific Diagram

Question Video Identifying The Products Of The Thermal Decomposition Of Ammonium Nitrite Nagwa

Solved Ammonium Nitrate Nh4no3 Decomposes Into Nitrogen Chegg Com
Comments
Post a Comment